SKN-1/Nrf and Stress Defenses
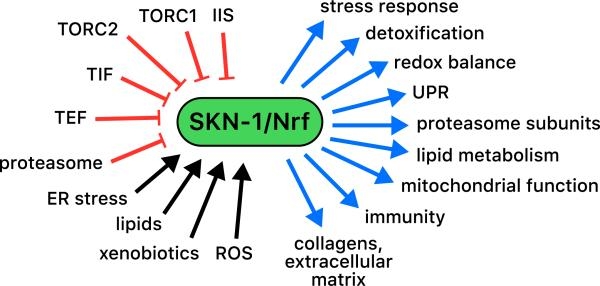
A remarkably complex network of regulatory pathways and protective mechanisms defend against metabolic and environmental stresses, and thereby maintain cellular and organismal homeostasis. Many of our studies of these processes are centered on the transcription factor SKN-1, which we showed several years ago is the C. elegans counterpart to the mammalian Nrf1/2/3 proteins. SKN-1 and Nrf proteins orchestrate a transcriptional response to oxidative and xenobiotic stress, but also have a broader range of functions that seem to be conserved. These functions include regulation of genes involved in metabolism, drug transport, protein homeostasis, and endoplasmic reticulum (ER) stress, among other important processes. The mechanisms that regulate SKN-1 in these contexts are more complex and nuanced than might be expected. For example, SKN-1 controls the major transcriptional response to proteasomal stress, but regulates different genes under conditions where protein synthesis is perturbed. A similar complexity of SKN-1 functions and regulation is seen when we compare its roles in oxidative stress, ER homeostasis, and metabolism. We are investigating at the biochemical, cellular, and organismal levels how signals and co-regulators control SKN-1 and other stress response regulators to achieve this level of complexity. We are also studying the “normal” functions of these stress defenses in maintaining health of the organism.
Longevity and Healthy Aging
In recent years it has become apparent that the process of aging is neither fixed nor random, and that essentially every organism has in place mechanisms that can dramatically alter its lifespan. We want to identify and understand these mechanisms, and develop paradigms through which they might eventually be harnessed to enhance human health and lifespan. Our work in C. elegans has revealed that SKN-1 and the processes it controls play a critical role in assuring longevity, and in the potential to delay aging. For example, across species life- and health-span can be increased by reducing the activity of insulin/IGF-1 signaling, an effect that was discovered in C. elegans. We have determined that SKN-1 is regulated by insulin/IGF-1 signaling, and plays a key role in the effects of this pathway on stress resistance and lifespan. SKN-1 is also critical in most other pathways or interventions that can be mobilized to extend the lifespan of C. elegans, including reducing activity of TOR (target of rapamycin) signaling, another growth regulatory pathway, and dietary restriction. Our lab investigates how such mechanisms that influence aging control SKN-1, and other regulators that it cooperates with. We also examine how these regulators act at the cellular and tissue level to promote longevity. We study important longevity-effector mechanisms, such as processes that maintain protein homeostasis or regulate metabolism, and how they influence organismal health. In addition, an emerging area of interest is in how longevity-promoting interventions not only protect cells but also influence the extracellular matrices in which they reside, and how this enhances health and lifespan. We expect that the fundamental mechanisms that we uncover in C. elegans will identify new areas of inquiry to be pursued in mammalian and human models.
|

|